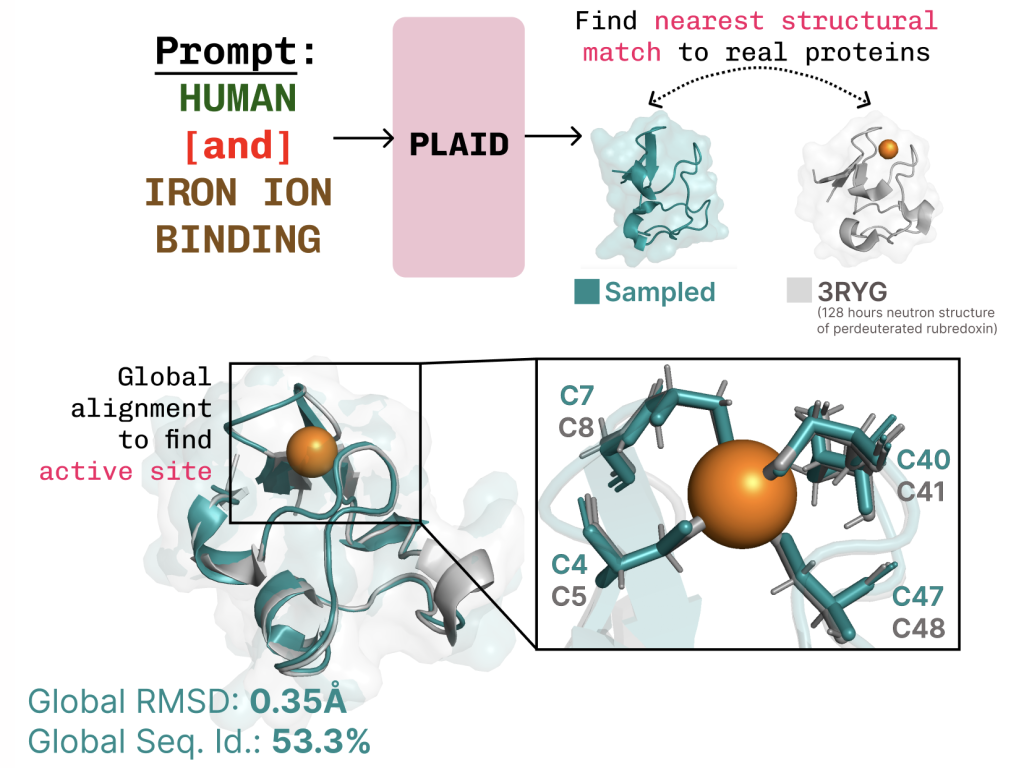
PLAID is a multimodal generative mannequin that concurrently generates protein 1D sequence and 3D construction, by studying the latent area of protein folding fashions.
The awarding of the 2024 Nobel Prize to AlphaFold2 marks an vital second of recognition for the of AI position in biology. What comes subsequent after protein folding?
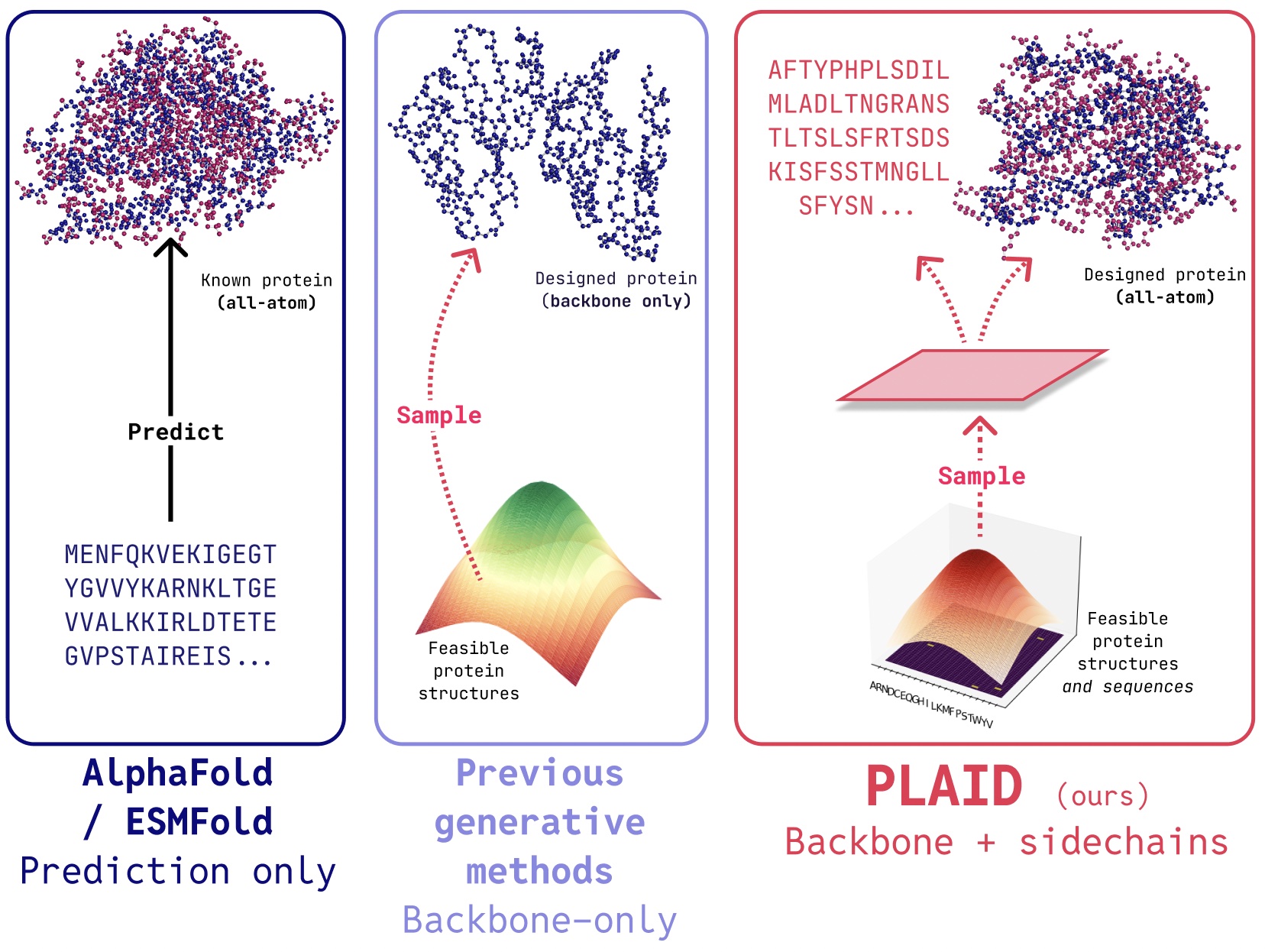
In PLAID, we develop a technique that learns to pattern from the latent area of protein folding fashions to generate new proteins. It will probably settle for compositional operate and organism prompts, and could be educated on sequence databases, that are 2-4 orders of magnitude bigger than construction databases. In contrast to many earlier protein construction generative fashions, PLAID addresses the multimodal co-generation downside setting: concurrently producing each discrete sequence and steady all-atom structural coordinates.
From construction prediction to real-world drug design
Although current works exhibit promise for the power of diffusion fashions to generate proteins, there nonetheless exist limitations of earlier fashions that make them impractical for real-world purposes, reminiscent of:
- All-atom era: Many present generative fashions solely produce the spine atoms. To supply the all-atom construction and place the sidechain atoms, we have to know the sequence. This creates a multimodal era downside that requires simultaneous era of discrete and steady modalities.
- Organism specificity: Proteins biologics meant for human use must be humanized, to keep away from being destroyed by the human immune system.
- Management specification: Drug discovery and placing it into the arms of sufferers is a posh course of. How can we specify these advanced constraints? For instance, even after the biology is tackled, you would possibly determine that tablets are simpler to move than vials, including a brand new constraint on soluability.
Producing “helpful” proteins
Merely producing proteins is just not as helpful as controlling the era to get helpful proteins. What would possibly an interface for this appear like?

For inspiration, let’s contemplate how we would management picture era by way of compositional textual prompts (instance from Liu et al., 2022).
In PLAID, we mirror this interface for management specification. The last word objective is to manage era totally by way of a textual interface, however right here we contemplate compositional constraints for 2 axes as a proof-of-concept: operate and organism:
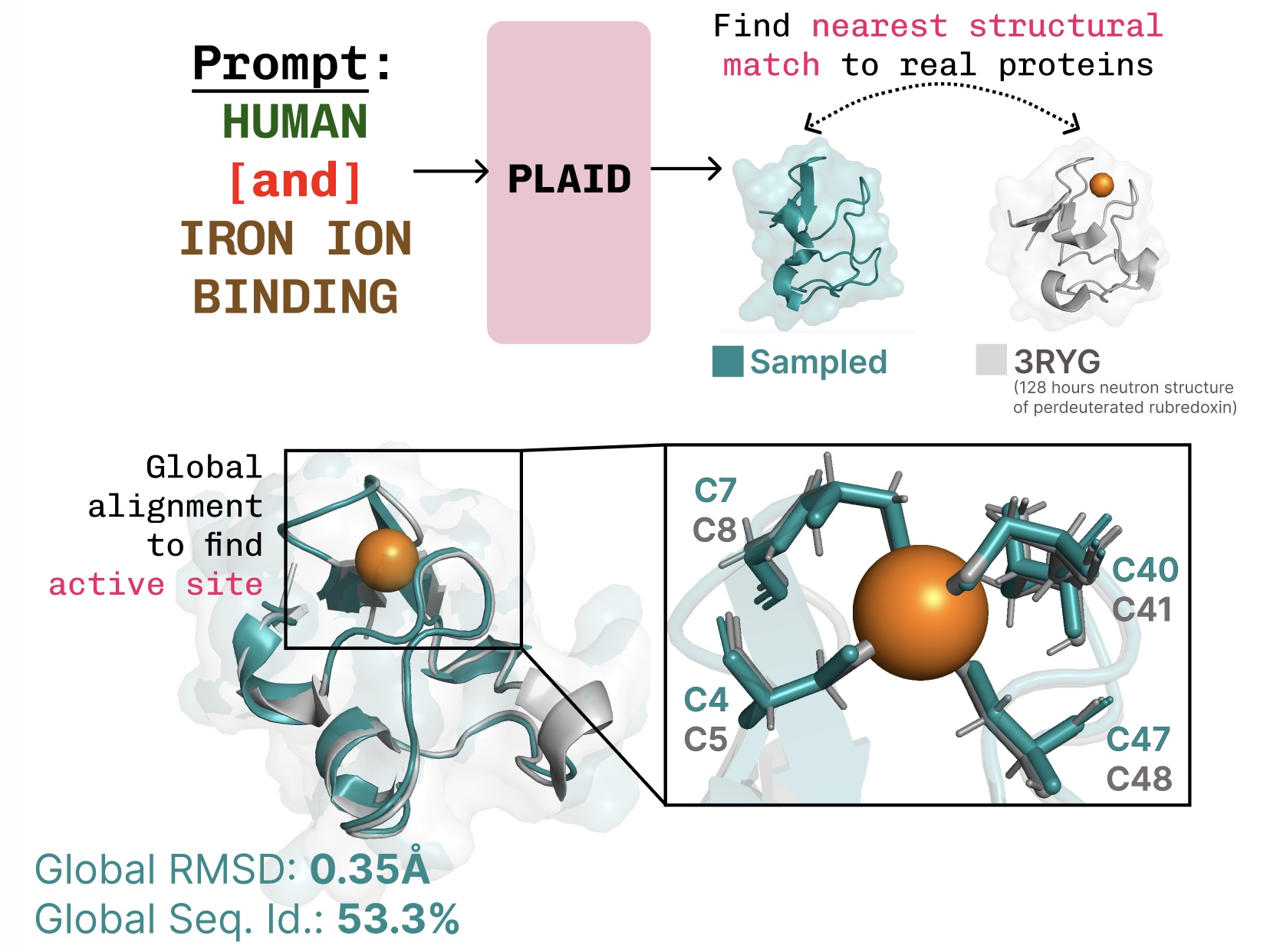
Studying the function-structure-sequence connection. PLAID learns the tetrahedral cysteine-Fe2+/Fe3+ coordination sample typically present in metalloproteins, whereas sustaining excessive sequence-level variety.
Coaching utilizing sequence-only coaching information
One other vital facet of the PLAID mannequin is that we solely require sequences to coach the generative mannequin! Generative fashions study the info distribution outlined by its coaching information, and sequence databases are significantly bigger than structural ones, since sequences are less expensive to acquire than experimental construction.
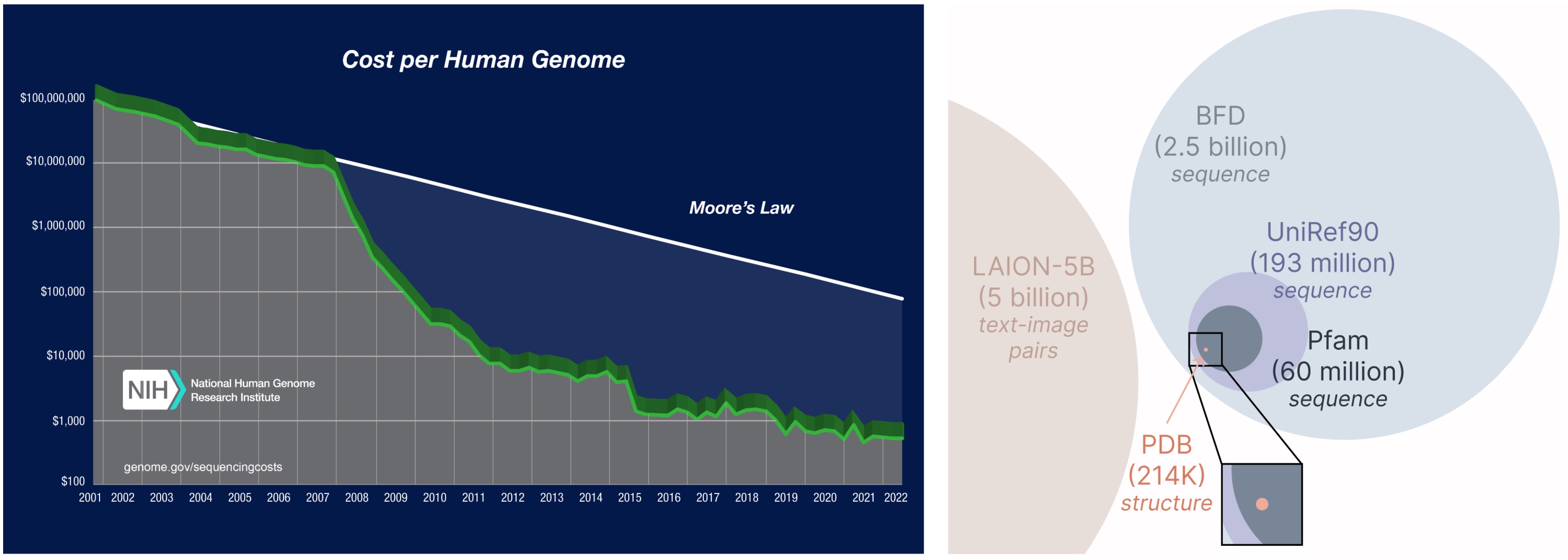
Studying from a bigger and broader database. The price of acquiring protein sequences is far decrease than experimentally characterizing construction, and sequence databases are 2-4 orders of magnitude bigger than structural ones.
How does it work?
The rationale that we’re capable of practice the generative mannequin to generate construction by solely utilizing sequence information is by studying a diffusion mannequin over the latent area of a protein folding mannequin. Then, throughout inference, after sampling from this latent area of legitimate proteins, we will take frozen weights from the protein folding mannequin to decode construction. Right here, we use ESMFold, a successor to the AlphaFold2 mannequin which replaces a retrieval step with a protein language mannequin.
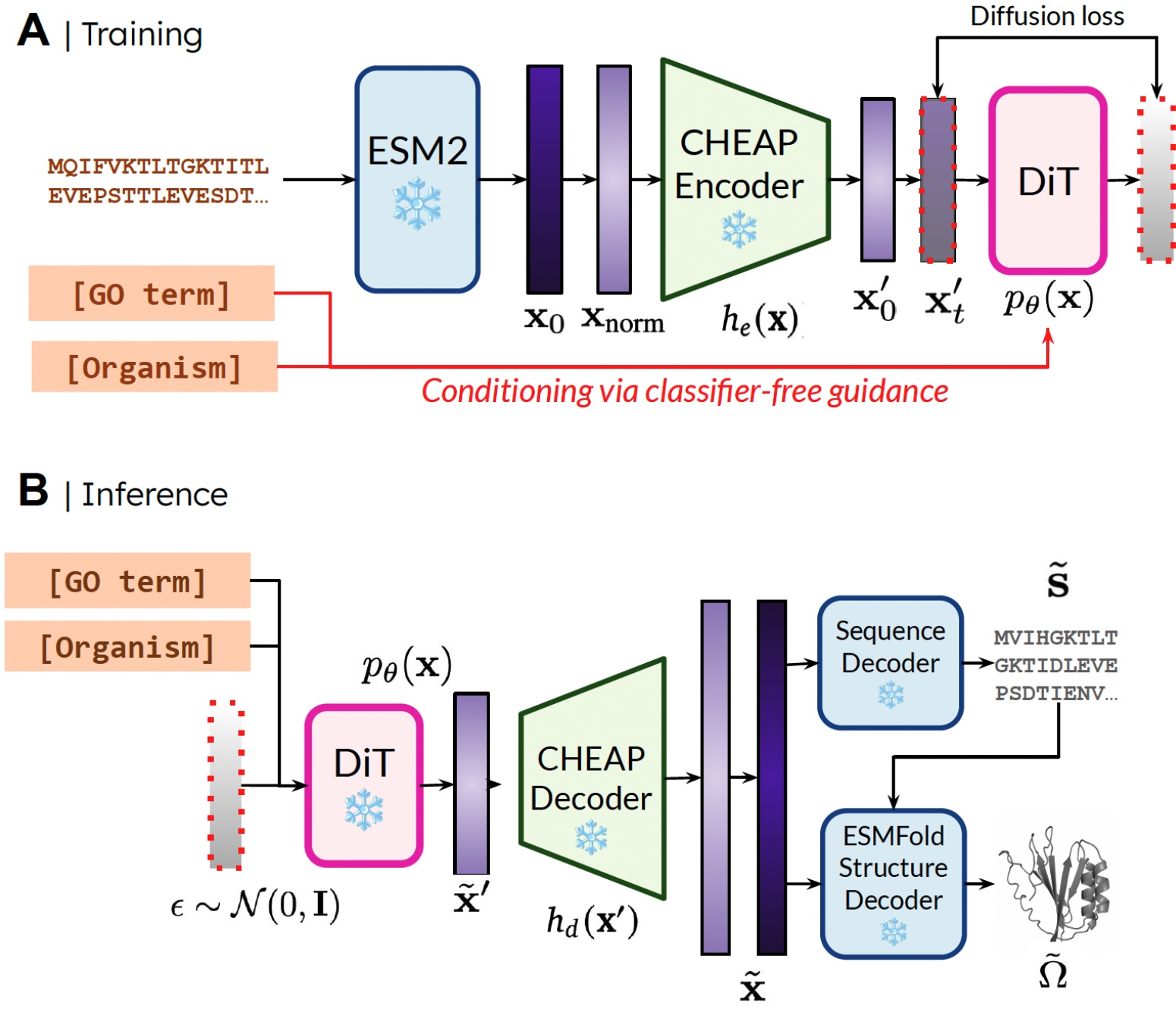
Our technique. Throughout coaching, solely sequences are wanted to acquire the embedding; throughout inference, we will decode sequence and construction from the sampled embedding. ❄️ denotes frozen weights.
On this approach, we will use structural understanding info within the weights of pretrained protein folding fashions for the protein design job. That is analogous to how vision-language-action (VLA) fashions in robotics make use of priors contained in vision-language fashions (VLMs) educated on internet-scale information to produce notion and reasoning and understanding info.
Compressing the latent area of protein folding fashions
A small wrinkle with instantly making use of this technique is that the latent area of ESMFold – certainly, the latent area of many transformer-based fashions – requires a whole lot of regularization. This area can be very giant, so studying this embedding finally ends up mapping to high-resolution picture synthesis.
To handle this, we additionally suggest CHEAP (Compressed Hourglass Embedding Variations of Proteins), the place we study a compression mannequin for the joint embedding of protein sequence and construction.
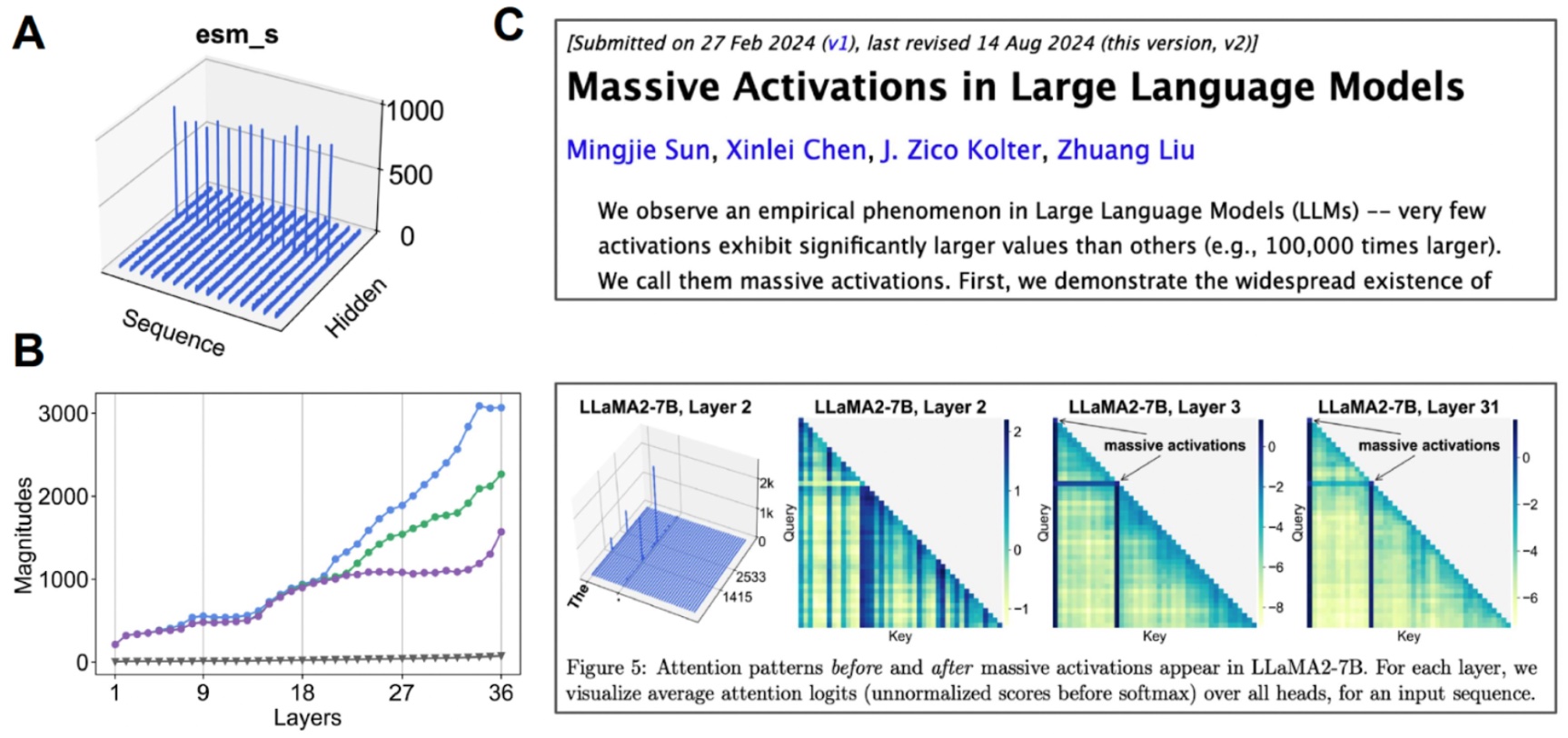
Investigating the latent area. (A) Once we visualize the imply worth for every channel, some channels exhibit “huge activations”. (B) If we begin analyzing the top-3 activations in comparison with the median worth (grey), we discover that this occurs over many layers. (C) Huge activations have additionally been noticed for different transformer-based fashions.
We discover that this latent area is definitely extremely compressible. By doing a little bit of mechanistic interpretability to higher perceive the bottom mannequin that we’re working with, we had been capable of create an all-atom protein generative mannequin.
What’s subsequent?
Although we study the case of protein sequence and construction era on this work, we will adapt this technique to carry out multi-modal era for any modalities the place there’s a predictor from a extra ample modality to a much less ample one. As sequence-to-structure predictors for proteins are starting to deal with more and more advanced methods (e.g. AlphaFold3 can be capable of predict proteins in advanced with nucleic acids and molecular ligands), it’s straightforward to think about performing multimodal era over extra advanced methods utilizing the identical technique.
In case you are considering collaborating to increase our technique, or to check our technique within the wet-lab, please attain out!
Additional hyperlinks
For those who’ve discovered our papers helpful in your analysis, please think about using the next BibTeX for PLAID and CHEAP:
@article{lu2024generating,
title={Producing All-Atom Protein Construction from Sequence-Solely Coaching Knowledge},
writer={Lu, Amy X and Yan, Wilson and Robinson, Sarah A and Yang, Kevin Okay and Gligorijevic, Vladimir and Cho, Kyunghyun and Bonneau, Richard and Abbeel, Pieter and Frey, Nathan},
journal={bioRxiv},
pages={2024--12},
12 months={2024},
writer={Chilly Spring Harbor Laboratory}
}
@article{lu2024tokenized,
title={Tokenized and Steady Embedding Compressions of Protein Sequence and Construction},
writer={Lu, Amy X and Yan, Wilson and Yang, Kevin Okay and Gligorijevic, Vladimir and Cho, Kyunghyun and Abbeel, Pieter and Bonneau, Richard and Frey, Nathan},
journal={bioRxiv},
pages={2024--08},
12 months={2024},
writer={Chilly Spring Harbor Laboratory}
}
It’s also possible to checkout our preprints (PLAID, CHEAP) and codebases (PLAID, CHEAP).
Some bonus protein era enjoyable!
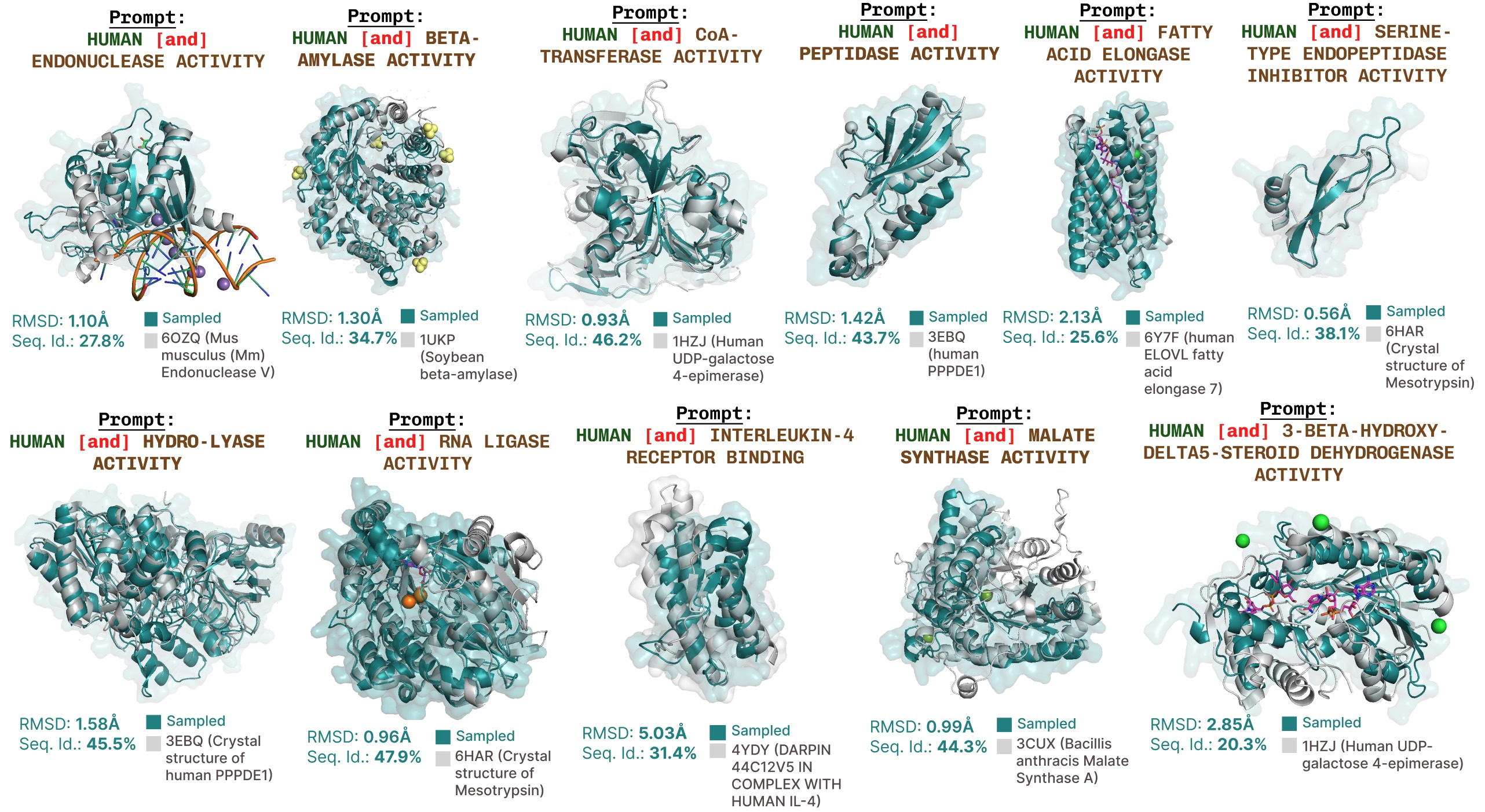
Further function-prompted generations with PLAID.
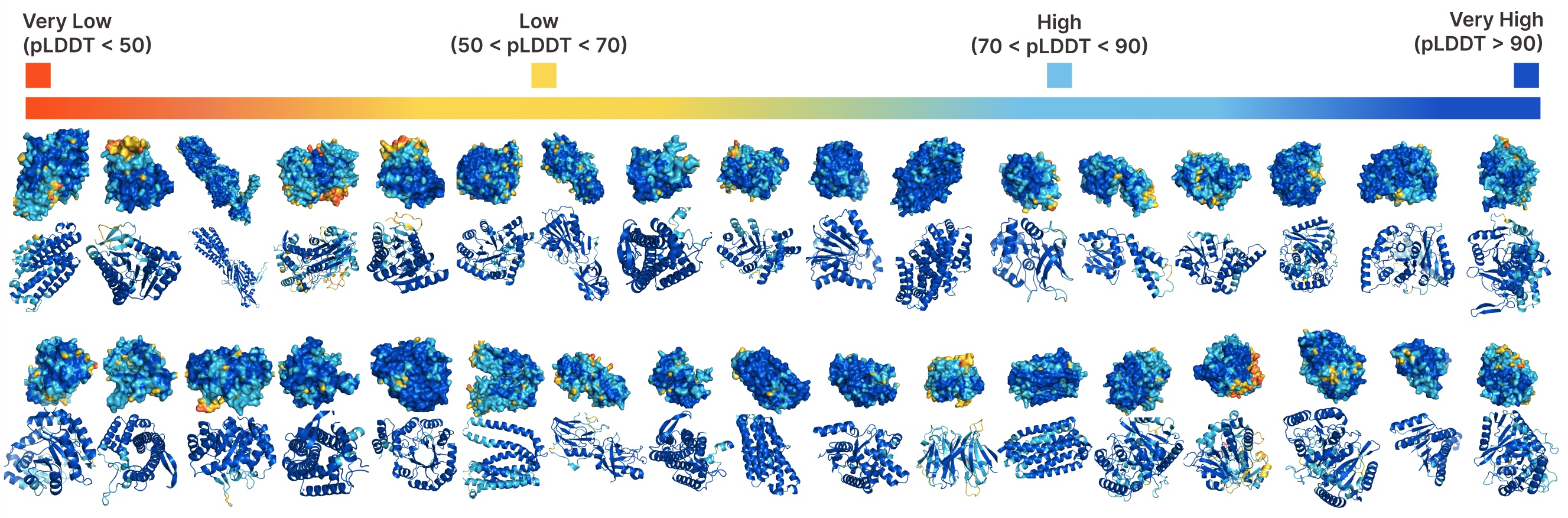
Unconditional era with PLAID.
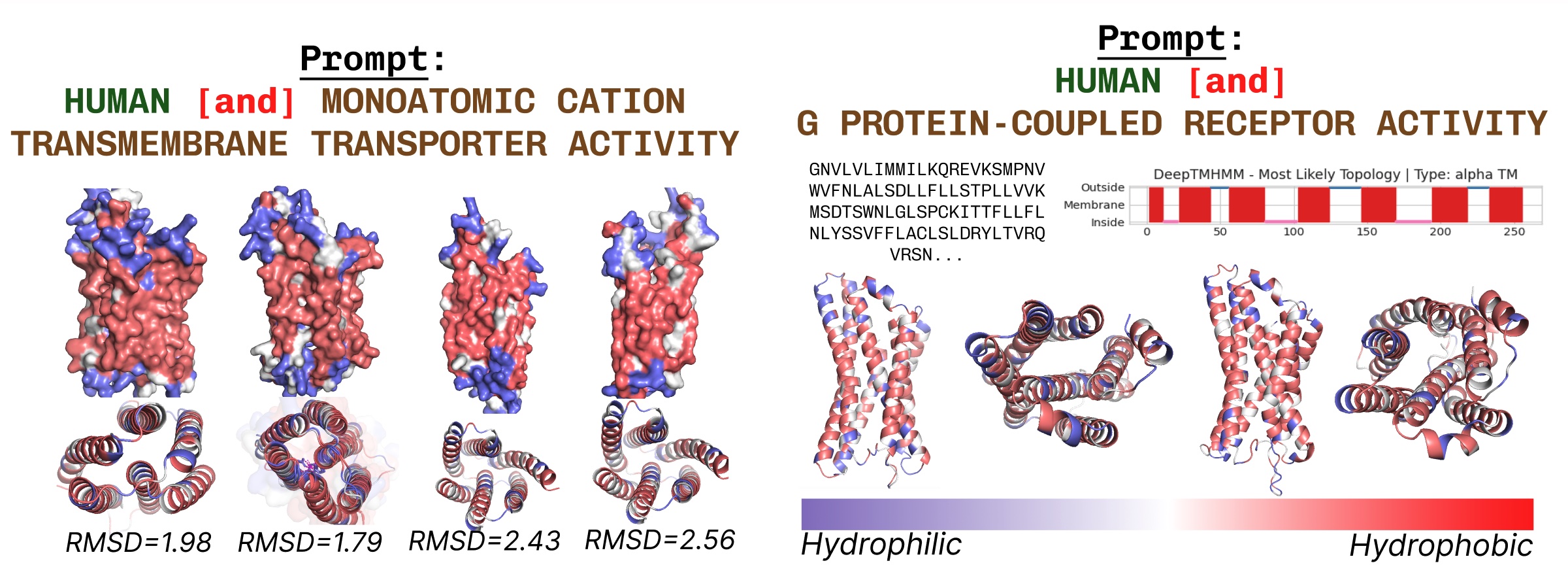
Transmembrane proteins have hydrophobic residues on the core, the place it’s embedded inside the fatty acid layer. These are persistently noticed when prompting PLAID with transmembrane protein key phrases.
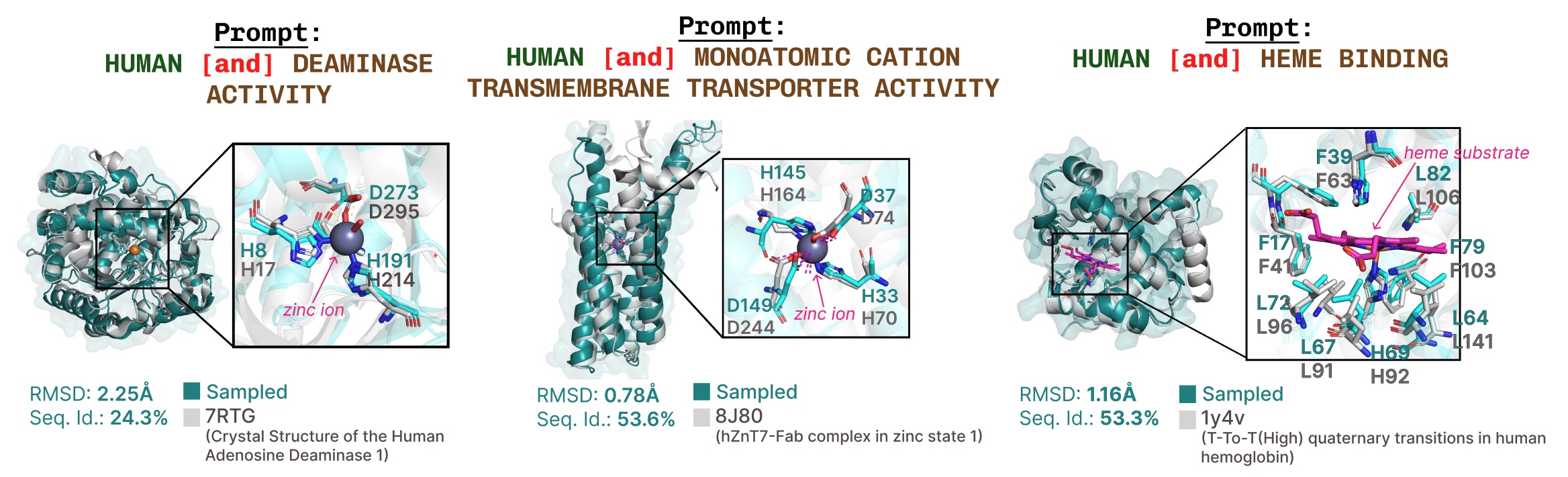
Further examples of lively web site recapitulation based mostly on operate key phrase prompting.
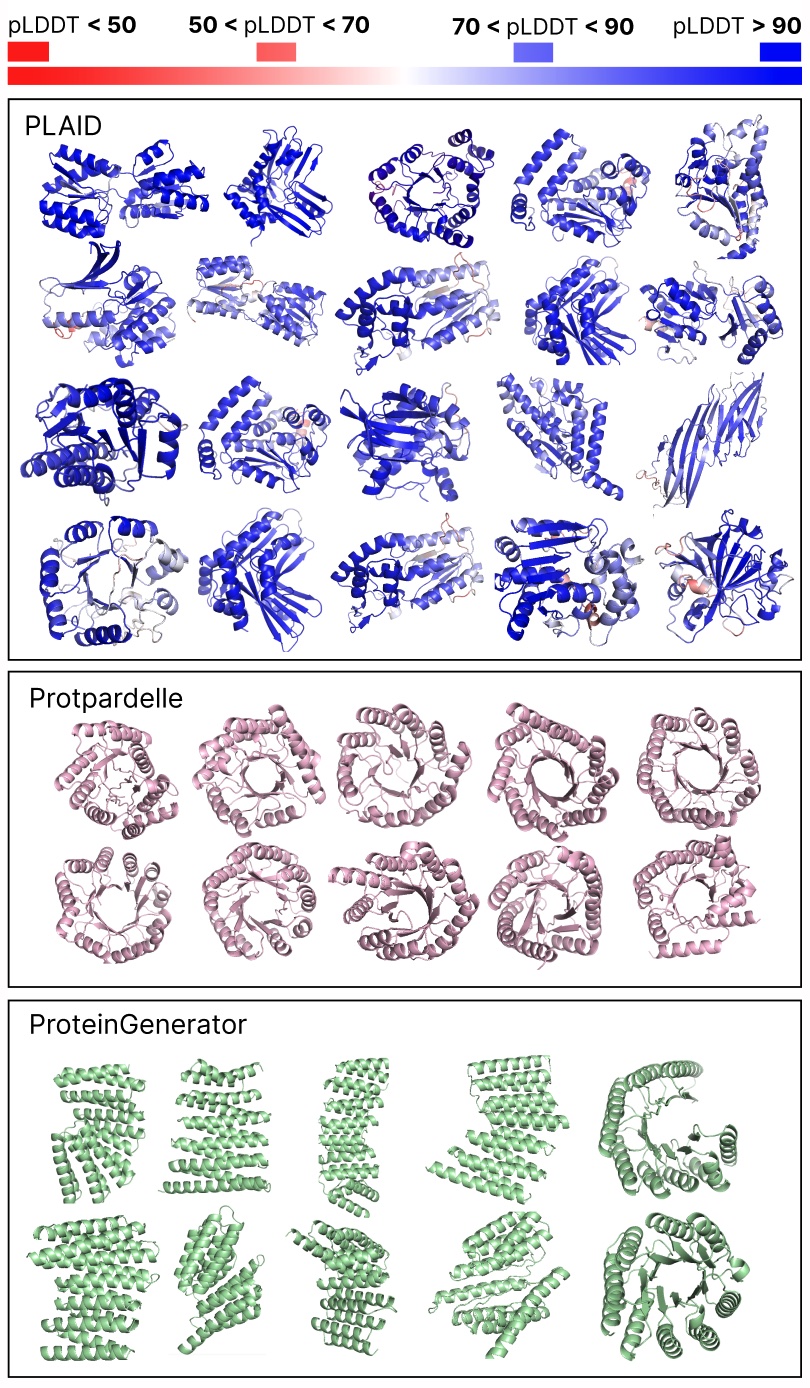
Evaluating samples between PLAID and all-atom baselines. PLAID samples have higher variety and captures the beta-strand sample that has been tougher for protein generative fashions to study.
Acknowledgements
Because of Nathan Frey for detailed suggestions on this text, and to co-authors throughout BAIR, Genentech, Microsoft Analysis, and New York College: Wilson Yan, Sarah A. Robinson, Simon Kelow, Kevin Okay. Yang, Vladimir Gligorijevic, Kyunghyun Cho, Richard Bonneau, Pieter Abbeel, and Nathan C. Frey.